Chinook Therapeutics to Present Updated Data from Zigakibart (BION-1301) Phase 1/2 Trial in Patients with IgA Nephropathy (IgAN) at the 60th European Renal Association (ERA) Congress
- Zigakibart treatment continues to demonstrate rapid and sustained reductions in mechanistic biomarkers, including IgA and Gd-IgA1 levels, which correspond to clinically meaningful proteinuria reductions in patients with IgAN across Cohorts 1 and 2
- Zigakibart is well-tolerated, with no ADAs observed or treatment discontinuations due to adverse events (AEs) in patients with IgAN across Cohorts 1 and 2
- In all patients combined from both Cohorts 1 and 2, zigakibart demonstrated mean proteinuria reductions of 20% at 12 weeks of treatment, 39% at 24 weeks of treatment and 67% at 52 weeks of treatment
- Extended treatment with zigakibart resulted in sustained clinical benefit, with 67% mean proteinuria reduction in seven patients at 76 weeks of treatment and 72% in five patients at 100 weeks of treatment
- Additional presentations on the phase 2 ASSIST and phase 3 BEYOND study designs, initial data from the phase 1 study of CHK-336 in healthy volunteers, as well as research on the impact of maladaptive tubular epithelial cells on disease progression in chronic kidney diseases will also be presented at the 60th ERA
Congress
- Due to the pending acquisition of
Chinook by Novartis AG, the investor conference call and webcast previously scheduled forFriday, June 16, 2023 at8:15 am EDT (2:15 pm CEST ) has been cancelled
“The strong data we will be presenting at the
Updated Interim Results of a Phase 1/2 Study of Zigakibart (BION-1301) in Patients with IgA Nephropathy
Zigakibart is a novel anti-APRIL monoclonal antibody currently in phase 2 clinical development for patients with IgAN. Blocking APRIL is a potentially disease-modifying approach to treating IgAN by reducing circulating levels of galactose-deficient IgA1 (Gd-IgA1).
Updated data from both Cohorts 1 and Cohort 2 will be presented from Part 3 of the ongoing phase 1/2 multi-center trial (see www.clinicaltrials.gov, identifier NCT03945318) evaluating the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD) and clinical responses of open-label zigakibart treatment in patients with IgAN.
Key highlights from the presentation include the following:
Patients in Cohort 1 initially received a 450mg intravenous (IV) dose of zigakibart every two weeks. After at least 24 weeks of IV dosing, patients in Cohort 1 transitioned to a 600 mg subcutaneous (SC) dose every two weeks for a total treatment duration of up to two years. Cohort 1 enrolled 10 patients, of which two patients withdrew from the study for reasons unrelated to study drug, and eight patients continued receiving treatment.
Patients in Cohort 2 are receiving a SC dose of 600 mg of zigakibart every two weeks for a total treatment duration of up to two years. Cohort 2 enrolled 30 patients, of which three patients were discontinued for not meeting the eligibility criterion of having biopsy-confirmed IgAN, and 27 patients continued receiving treatment.
Baseline 24-hour Urine Protein Excretion (g/day)
- The median baseline 24-hour urine protein excretion for patients enrolled in Cohort 1 was 1.2 g/day, with a range of 0.7 – 6.5 g/day, and the median baseline 24-hour urine protein excretion for patients enrolled in Cohort 2 was 1.1 g/day, with a range of 0.3 – 7.0 g/day. With a median baseline 24-hour urine protein excretion for patients enrolled in both Cohorts 1 and 2 of 1.1 g/day, this population represents patients with IgAN at high risk of kidney disease progression.
Safety and Tolerability
- As of the
May 8, 2023 data cutoff, zigakibart has been well tolerated, with no deaths or treatment discontinuations due to adverse events. Of all 40 patients enrolled in both Cohorts 1 and 2:
- All infections have been Grade 1 or 2 in severity and only one subject had infections deemed treatment-related (Grade 1 viral upper respiratory tract infection and influenza).
- There were no anti-drug antibodies observed in any patients.
- Two patients had IgG levels that fell below 3 g/
L. One patient in Cohort 1 required protocol-mandated withholding of study drug. The patient reached end-of-treatment prior to re-initiation of study drug. One patient in Cohort 2 had IgG levels below 3g/L at their week 12 follow-up after permanent discontinuation due to not meeting eligibility criteria for having biopsy-confirmed IgAN. No infections were reported in either patient while their IgG levels were below 3g/L.
- There were 16 injection site reactions (ISRs) reported from a total of 875 SC doses administered (<2%). All ISRs were Grade 1 or Grade 2.
- One serious adverse event occurred (amnesia) that was not treatment-related and did not result in interruption of study drug.
- All infections have been Grade 1 or 2 in severity and only one subject had infections deemed treatment-related (Grade 1 viral upper respiratory tract infection and influenza).
Mechanistic Biomarkers
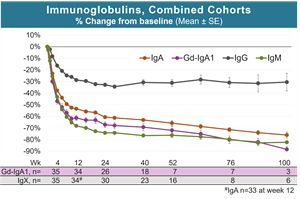
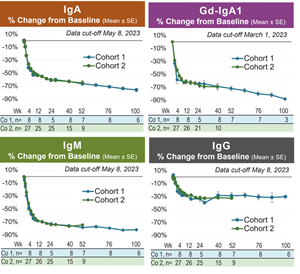
- Zigakibart treatment resulted in rapid and sustained reductions in IgA, pathogenic Gd-IgA1, IgM and to a lesser extent IgG, in patients with IgAN. Zigakibart’s effects on mechanistic biomarkers were highly consistent between Cohorts 1 and 2 (see figures below).
https://www.globenewswire.com/NewsRoom/AttachmentNg/6608e190-53ad-4117-b6da-e521a2f9acef
https://www.globenewswire.com/NewsRoom/AttachmentNg/baac3459-0bf7-4b0d-8ffe-36e05158951f
24-hour UPCR
- Zigakibart treatment resulted in sustained, clinically meaningful proteinuria reductions in patients with IgAN across a wide range of baseline proteinuria levels.
- In the combined Cohorts 1 and 2, zigakibart demonstrated mean reductions in 24-hour urine protein creatinine ratio (UPCR) of 20% in 33 patients at 12 weeks of treatment, 39% in 33 patients at 24 weeks of treatment, 67% in 17 patients at 52 weeks of treatment, 67% in seven patients at 76 weeks of treatment and 72% in five patients at 100 weeks of treatment (see figure below).
https://www.globenewswire.com/NewsRoom/AttachmentNg/dca0b8df-b710-4f2c-b410-33f8aeafaf01
Overviews of the phase 3 BEYOND and phase 2 ASSIST trials are also being presented as focused oral presentations (digital poster with 3-minute presentation) on
Focused Orals:
| Abstract Title: | Updated Interim Results of a Phase 1/2 Study of BION-1301 in Patients with IgA Nephropathy |
| Presenting Author: | |
| Session: | Glomerular & Tubulo-interstitial Diseases |
| Date/Time: | |
| Location: | |
| Abstract Title: | A Phase 3, Randomized, Double-blind, Placebo-controlled Study of BION-1301 in Adults with IgA Nephropathy |
| Presenting Author: | |
| Session: | Glomerular & Tubulo-interstitial Diseases |
| Date/Time: | |
| Location: | |
| Abstract Title: | ASSIST Study Design: A Randomized, Double-blind, Placebo-controlled, Crossover Study of Atrasentan in Patients with IgA Nephropathy (IgAN) on Sodium-glucose Cotransporter-2 Inhibitors (SGLT2i) |
| Presenting Author: | Hiddo J. L Heerspink, PhD, PharmD |
| Session: | Glomerular & Tubulo-interstitial Diseases |
| Date/Time: | |
| Location: |
Free Communication:
| Abstract Title: | CHK-336, A First-in-Class Orally Administered LDH Inhibitor: Safety, PK and Target Engagement in a First-in-Human Phase 1 Healthy Volunteer Study |
| Presenting Author: | |
| Session: | Something Rare, Something Special |
| Date/Time: | |
| Location: | Amber 3 & 4 |
Moderated Oral:
| Abstract Title: | Accumulation of Maladaptive Tubular Epithelial Cells (TECs) is Ubiquitous in Chronic Kidney Diseases and Represents a Common Initiating Mechanism of Disease Progression |
| Presenting Author: | |
| Session: | Moderated Orals 1.4 |
| Date/Time: | |
| Location: | Amber 6 |
Once presented, all five presentations can be found in the
Due to the pending acquisition of
About Chinook Therapeutics, Inc.
Forward-Looking Statements
In addition to historical information, this communication contains forward-looking statements within the meaning of applicable securities law, including statements regarding the advancement of its product candidates and product pipeline, and the clinical development of its product candidates, including expectations regarding the results of clinical trials. In addition, when used in this communication, the words “will,” “expects,” “could,” “would,” “may,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “targets,” “estimates,” “looks for,” “looks to,” “continues” and similar expressions, as well as statements regarding our focus for the future, are generally intended to identify forward-looking statements. Each of the forward-looking statements we make in this communication involves risks and uncertainties that could cause actual results to differ materially from these forward-looking statements. Factors that might cause or contribute to such differences include, but are not limited to: expected revenues, cost savings, synergies and other benefits from the proposed merger might not be realized within the expected time frames or at all and costs or difficulties relating to integration matters, including but not limited to employee retention, might be greater than expected; the requisite regulatory approvals and clearances for the proposed transaction may be delayed or may not be obtained (or may result in the imposition of conditions that could adversely affect the combined company or the expected benefits of the proposed merger); the requisite approval of Company stockholders may be delayed or may not be obtained, the other closing conditions to the proposed merger may be delayed or may not be obtained, or the merger agreement may be terminated; business disruption may occur following or in connection with the proposed merger; Novartis or Chinook’s businesses may experience disruptions due to transaction-related uncertainty or other factors making it more difficult to maintain relationships with employees, other business partners or governmental entities; the milestones for the proposed CVRs may not be achieved; the possibility that the proposed merger is more expensive to complete than anticipated, including as a result of unexpected factors or events; and diversion of management’s attention from ongoing business operations and opportunities as a result of the proposed merger or otherwise. Additional factors that may affect the future results of Novartis and
Additional Information and Where to Find It
In connection with the proposed merger between Novartis and
Participants in the Solicitation
This document does not constitute a solicitation of proxy, an offer to purchase or a solicitation of an offer to sell any securities. Novartis,

Investor Contact:Noopur Liffick , MPH Senior Vice President, Investor Relations & Corporate Communications investors@chinooktx.com Media Contact:Kelly North Senior Manager, Investor Relations & Corporate Communications media@chinooktx.com
Zigakibart Treatment Results in Rapid and Sustained Reductions in IgA and Gd-IgA1 in Patients with IgA Nephropathy
Zigakibart produced rapid and sustained reductions in IgA and Gd-IgA1, the pathogenic variant of IgA nephropathy
Zigakibart Treatment Results in Rapid and Sustained Reductions in IgA and Gd-IgA1 in Patients with IgA Nephropathy
Zigakibart produced rapid and sustained reductions in IgA and Gd-IgA1, the pathogenic variant of IgA nephropathy
Zigakibart Treatment Results in Clinically Meaningful Proteinuria Reductions in Patients with IgAN
Zigakibart treatment resulted in sustained, clinically meaningful proteinuria reductions in patients with IgA nephropathy across a wide range of baseline proteinuria levels
Source: Chinook Therapeutics, Inc.