UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of report (Date of earliest event reported): January 13, 2020
ADURO BIOTECH, INC.
(Exact name of Registrant as Specified in its Charter)
| Delaware | 001-37345 | 94-3348934 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
740 Heinz Avenue
Berkeley, California 94710
(Address of Principal Executive Offices) (Zip Code)
Registrant’s telephone number, including area code: (510) 848-4400
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communication pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
| Common Stock, par value $0.0001 per share | ADRO | The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
| Item 8.01. | Other Events. |
Attached hereto as Exhibit 99.1 is an investor presentation that Aduro Biotech, Inc. plans to present during the 38th Annual J.P. Morgan Healthcare Conference commencing on January 13, 2020.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit No. |
Description | |
| 99.1 | Aduro Biotech, Inc. Investor Presentation. | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| ADURO BIOTECH, INC. | ||||||
| Dated: January 13, 2020 | By: | /s/ Celeste Ferber | ||||
| Name: | Celeste Ferber | |||||
| Title: | SVP, General Counsel and Secretary | |||||

Corporate Presentation January 2020 DISCOVERING & DEVELOPING THERAPIES FOR CHALLENGING DISEASES Exhibit 99.1

Note Regarding Forward-Looking Statements This presentation and the accompanying oral presentation include express and implied forward-looking statements regarding the current intentions, expectations, estimates, opinions and beliefs of Aduro Biotech, Inc. (“Aduro”) that are not historical facts. These forward-looking statements include statements regarding Aduro’s expectations for its product candidates (including their therapeutic and commercial potential, anticipated future development activities, anticipated timing of development activities, including initiation of clinical trials and presentations of clinical data and the indications Aduro and its collaborators plan to pursue), future results of operations and financial position, including current funds providing operating capital into 2022 and the recent restructuring resulting in reductions in operating expenses and an extended cash runway, business strategy, strategic collaborations, any royalty or milestone payments and Aduro’s ability to obtain and maintain intellectual property protection for its product candidates. Such forward-looking statements may be identified by words such as “believes,” “may,” “will,” “expects,” “endeavors,” “anticipates,” “intends,” “plans,” “estimates,” “projects,” “should,” “objective” and variations of such words and similar words. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, Aduro’s history of net operating losses and uncertainty regarding its ability to achieve profitability, Aduro’s ability to develop and commercialize its product candidates, Aduro’s ability to use and expand its technology to build a pipeline of product candidates, Aduro’s ability to obtain and maintain regulatory approval of its product candidates, Aduro’s ability to operate in a competitive industry and compete successfully against competitors that have greater resources than it does, Aduro’s reliance on third parties, Aduro’s ability to execute on its corporate strategy, including the success of the restructuring and Aduro’s ability retain senior management and other highly qualified personnel, and Aduro’s ability to obtain and adequately protect intellectual property rights for its product candidates. Aduro discusses many of these risks in greater detail under the heading "Risk Factors" in its most recent Quarterly or Annual Report on Form 10-Q or Form 10-K filed with the Securities and Exchange Commission. Any forward-looking statements that Aduro or any presenter makes in this presentation and the accompanying oral presentation speak only as of the date of these presentations. Except as required by law, Aduro assumes no obligation to update its forward-looking statements whether as a result of new information, future events or otherwise, after the date hereof. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or Aduro or any director, employee, agent or adviser of Aduro. This presentation does not purport to be all-inclusive or to contain all of the information you may desire. The content of this presentation is subject to copyright, which will be asserted by Aduro, and no part of this presentation may be reproduced, stored in a retrieval system, or transmitted in any form or by any means without prior permission in writing from Aduro.
Developing Therapies Targeting STING & APRIL Pathways for Oncology, Autoimmune & Inflammatory Diseases Financial Strength Strategic Alliances Leaders in cGAS-STING & APRIL Pathways Co-development and co-commercialization partnership with NVS Potential near-term development milestones $235M at end of Q3 2019 Operating capital into 2022 STING agonist in oncology cGAS-STING inhibitors in autoimmune & inflammatory diseases Anti-APRIL antibody in IgA nephropathy Comprehensive preclinical characterization

Aduro Pipeline Indication Discovery Preclinical Phase 1 Phase 2 Current Status ADU-S100 (MIW815) Intratumoral STING Agonist Advanced/metastatic solid tumors/lymphomas Dose escalation completed No single agent expansion planned + spartalizumab (α-PD-1) Advanced/metastatic solid tumors/lymphomas Dose escalation completed Enrollment terminated No expansion planned + ipilimumab (α-CTLA-4) α-PD-1-relapsed-refractory melanoma Enrollment terminated + pembrolizumab (α-PD-1) 1st-line SCCHN Enrollment ongoing ADU-S100 Intravesical BCG-refractory NMIBC IND-enabling studies ongoing Systemic STING Agonists Oncology Jointly pursuing with Novartis cGAS-STING Inhibitors Autoimmune & inflammatory disorders Advancing compounds BION-1301 Anti-APRIL IgA nephropathy All SAD cohorts cleared Currently evaluating 3rd MAD cohort SCCHN = squamous cell carcinoma of the head and neck; BCG = Bacillus Calmette-Guerin; NMIBC = non-muscle invasive bladder cancer

ADU-S100 (MIW815) Intratumoral STING agonist

ADU-S100 (MIW815) Demonstrated Preclinical Anti-Tumor Activity STING activation in the hematopoietic compartment drives ADU-S100 (MIW815) dependent anti-tumor immunity Type I IFN is required for optimal anti-tumor immune response ADU-S100 (MIW815) induces both local innate immune activation in injected tumors (cytokine production) and activates tumor specific CD8+ T cells Bridging innate to adaptive immunity Dose level impacts induction of ADU-S100 (MIW815) tumor-specific T cell response Immunogenic dosing regimens induce systemic and durable adaptive tumor immunity ADU-S100 (MIW815) is an effective combination agent with checkpoint inhibition to enhance anti-tumor efficacy and durable immunity Corrales & Gajewski, Clin. Cancer Res (2015)

ADU-S100 (MIW815) Monotherapy
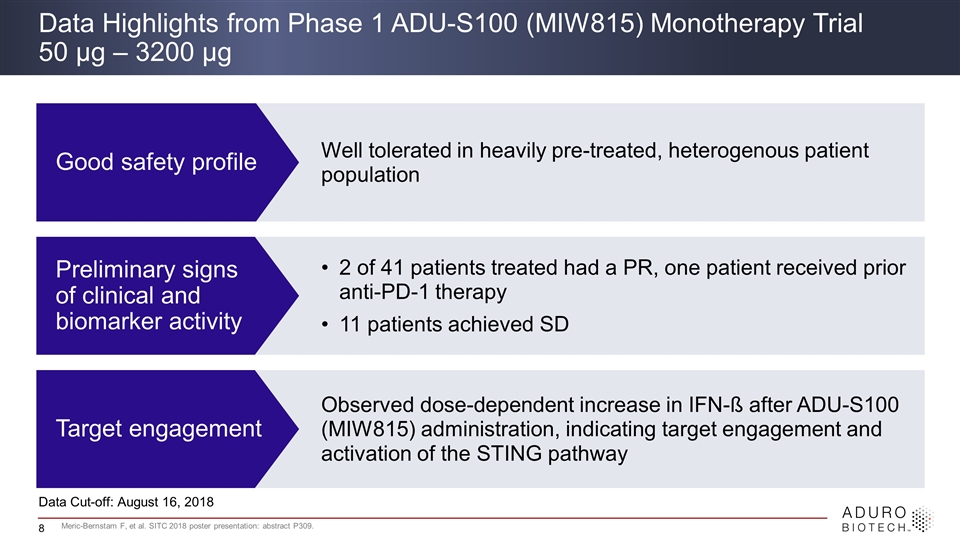
Well tolerated in heavily pre-treated, heterogenous patient population 2 of 41 patients treated had a PR, one patient received prior anti-PD-1 therapy 11 patients achieved SD Observed dose-dependent increase in IFN-ß after ADU-S100 (MIW815) administration, indicating target engagement and activation of the STING pathway Data Highlights from Phase 1 ADU-S100 (MIW815) Monotherapy Trial 50 μg – 3200 μg Good safety profile Preliminary signs of clinical and biomarker activity Target engagement Data Cut-off: August 16, 2018 Meric-Bernstam F, et al. SITC 2018 poster presentation: abstract P309.

ADU-S100 (MIW815) + spartalizumab (α-PD-1)

Preliminary Anti-tumor Activity CRC, colorectal cancer; SCC, squamous cell carcinoma. Data cut-off: April 5, 2019 No patients achieved a response; however, six patients achieved SD Tumor types: Ovarian, breast, uveal melanoma, head and neck, and cutaneous melanoma Four of whom maintained SD for ≥6 months ADU-S100 (MIW815) monthly Five confirmed responses (4 PR + 1 CR) Two PR and one CR in patients with IO-naive TNBC At time of data cut, these patients were on treatment for >6 months Two of these patients expressed PD-L1 levels of >1% at baseline (data from third TNBC patient not available) Two PR in patients with previously IO-treated melanoma Additional 12 patients achieved SD Tumor types: Sarcoma, melanoma, SCC skin, breast, lymphoma, and head and neck ADU-S100 (MIW815) 3-weeks-on/1-week-off Meric-Bernstam F, et al. ASCO 2019 oral presentation: abstract 2507.

ADU-S100 (MIW815) + spartalizumab was generally well tolerated in patients with solid tumors or lymphomas, with no DLTs reported as of the data cut-off The MTD was not reached The combination has demonstrated anti-tumor activity in PD-1–naive TNBC and PD-1–relapsed/refractory melanoma Three out of eight evaluable anti-PD-1-naïve-TNBC patients had a response (2 PRs + 1 CR) Two out of 25 evaluable melanoma patients had a response (2 PRs) ADU-S100 plasma exposure increases dose-proportionally IFN-β concentrations appeared to increase with increasing exposure to ADU-S100 (MIW815) Data Highlights from Phase 1 ADU-S100 (MIW815) + Spartalizumab 50 μg – 1600 μg Good safety profile Preliminary signs of clinical and biomarker activity Target engagement Data Cut-off: April 5, 2019 Meric-Bernstam F, et al. ASCO 2019 oral presentation: abstract 2507.

ADU-S100 (MIW815) + pembrolizumab (α-PD-1) in First-Line SCCHN

Phase 2 Ongoing: ADU-S100 (MIW815) + Pembrolizumab in First-Line SCCHN Shift from heavily pre-treated, heterogenous patient populations to earlier lines of treatment for patients with specific tumor type Increasing evidence that potential benefit from immunotherapies may be greater in patients with fewer prior therapies Advance to N = 34 based on ORR for N = 20 (Simon 2-stage criteria) Looking for an approximate doubling of the response rate with pembrolizumab alone: ORR for pembrolizumab in KEYNOTE-048: 19% in patients with CPS >1 23% in patients with CPS >20 Merck & Co., Inc. (2019, June 11). FDA Approves Two New Indications for Merck’s KEYTRUDA® (pembrolizumab) [Press Release]. Retrieved from https://investors.merck.com/news/press-release-details/2019/FDA-Approves-Two-New-Indications-for-Mercks-KEYTRUDA-pembrolizumab/default.aspx

Phase 2 Clinical Trial Design: ADU-S100 (MIW815) + Pembrolizumab in First-Line SCCHN Treatment Period ADU-S100: Fixed dose IT injection, up to two lesions Pembrolizumab: 200 mg IV infusion Patients are treated until confirmed disease progression, unacceptable toxicity or 35 cycles Phase 2 (N=34) Patients: Adults with recurrent or metastatic SCCHN, first-line setting Primary Objective: Evaluation of clinical activity by ORR per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 Key Inclusion Criteria: Histological or cytological confirmation of recurrent or metastatic SCCHN Measurable disease as defined by RECIST v1.1 PD-L1 positive (CPS >1) Key Exclusion Criteria: Diagnosis of recurrent or metastatic carcinoma of the nasopharynx, squamous cell carcinoma of unknown primary histology; or salivary gland or non-squamous histologies Disease amenable to local therapy with curative intent Prior systemic anti-cancer therapy for the treatment of recurrent or metastatic HNSCC Screening Period Follow-up Period Safety reporting Subsequent cancer treatment Survival

ADU-S100 (MIW815) Intravesical administration in BCG- refractory NMIBC

Rationale for Intravesical ADU-S100 in NMIBC Scientific Local, superficial disease in which local control drives disease outcome Ablation of the tumor may be sufficient to control disease, anenestic response less important Similar to BCG, ADU-S100 is a small molecule with potential to induce a local innate immune response BCG refractory patients have a TME enriched with ADU-S100 targets Mechanisms leading to BCG refractory state may not interfere with response to ADU-S100, e.g., M2 macrophages, receptor loss Clinical Development Potential for single agent therapeutic application of ADU-S100 Intravesical administration attractive alternative to systemic therapy BCG-unresponsive patients constitute a high unmet medical need recognized by the FDA with approval pathway outlined in guidance

Bladder Cancer is a “Hot” Tumor with High TMB & Inflammatory GEP Schumacher et al. Science. 2015. High mutational burden rate for potential immunogenicity

Scientific Rationale for ADU-S100 in BCG refractory NMIBC 4. Relatively high mutational burden rate, potential immunogenicity CD8+ T cell activation ADU-S100 1. BCG refractory patients have increased macrophages, S100 targets and turns on cytokine production 2. ADU-S100 is a strong innate immune stimulant that may drive the exfoliation response induced by infection/BCG 3. Bladder tumor cells have increased expression of a transporter for ADU-S100 compared to normal cells, may cause selective tumor killing

ADU-S100 Activates an Immune Response in the Bladder after Intravesicular Delivery and Controls Tumors in Preclinical Models Preclinical efficacy in standard orthotopic model, MB49 bladder tumor Observe local cytokine induction with intravesicular delivery of ADU-S100 Preliminary data demonstrates anti-tumor efficacy Orthotopic Bladder Tumor MB49 PBS 24 Hrs post TX: ADU-S100 50 µg Cytokines in Urine IP10 Relative protein levels 1 3 24 5

Major Unmet Need in BCG-Unresponsive NMIBC BCG treatment will eventually fail in up to 50% of patients Due to worldwide shortage, only 50% of patients who need BCG receive treatment Global Incidence of Bladder Cancer BCG-Relapsed/ Refractory Patients* Kamat, A., Flaig, T., Grossman, H. et al. Consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nat Rev Urol 12, 225–235 (2015) doi:10.1038/nrurol.2015.58

Valrubicin is the Only Approved Option for BCG Refractory CIS when Cystectomy is Not an Option and Few Ongoing Clinical Studies Single-arm study of patients with BCG failure 70% with ≥2 prior BCG courses 80% with 2 – 24 month interval between last treatment and valrubicin DFR 6 months = 21% Suboptimal salvage therapy for BCG failure

BION-1301 Anti-APRIL Antibody

IgA Nephropathy: Biology IgA nephropathy (IgAN) is a chronic autoimmune disease associated with inflammation in the kidneys that diminishes their ability to filter blood A key breakthrough was identification of IgA1 molecules lacking glycosylation at the hinge region, often referred to as galactose-deficient IgA1 (Gd-IgA1) In patients with IgAN, Gd-IgA1 gives rise to autoantibody production Gd-IgA1–autoantibody complexes deposit in the kidneys, resulting in complement activation, inflammation and subsequent renal damage

IgA Nephropathy: Epidemiology IgAN is the most frequent biopsy-proven primary glomerular disease, but the geographic distribution varies widely Clinical findings that trigger biopsy vary by country Biopsy registry data underestimate disease burden as patients with mild disease may not undergo biopsy, and in countries lacking screening programs, disease may not be detected Because the diagnosis of IgAN requires a kidney biopsy, the exact disease prevalence remains difficult to establish US prevalence is estimated to be ~130,000 – 150,000 UK prevalence is estimated to be ~10,000 – 15,000 Asian populations have a higher incidence rate of IgAN, with ~75,000 new cases diagnosed annually in China Schena FP, Nistor I, Semin Nephrol 2018, 38(5):435-442; Magistroni et al., Kidney Intl (2015) 88:974-89; Ilyas M. Pediatric IgA nephropathy. Medscape. 2017. https://emedicine.medscape.com/article/981516-overview.

IgA Nephropathy: Diagnosis & Disease Progression Diagnosis Patients often diagnosed in their 20s or 30s Disease Progression 30% to 40% of patients with IgAN progress to ESRD within approximately 20 years Diagnosis, which requires biopsy , often made following GI or respiratory infection with observation of macroscopic hematuria When screened, up to half of patients with microscopic hematuria found to have IgAN Routine screening of asymptomatic patients results in a high % of patients with IgAN Key risk factors for progression are blood pressure, proteinurea , kidney histology and eGFR High-risk patients – based on blood pressure, proteinuria and eGFR – have significantly increased mortality

IgA Nephropathy: Treatment There are no approved treatments for IgAN Current standard of care treatment includes: Controlling hypertension (via renin-angiotensin blockade), which can reduce proteinurea and slow disease progression Immunosuppressive drugs (e.g., corticosteroids for 6 months), which have very severe and long-lasting side effects and inconclusive long-term benefits Fish oil and tonsillectomy (in Japan), which have shown inconclusive benefit A significant portion of patients will progress despite blood pressure control No treatment available that selectively reduces the production of Gd-IgA1 and the anti-Gd-IgA autoantibodies that give rise to IgAN The need for an effective treatment for IgA Nephropathy is clear

APRIL and its Role in IgAN A Proliferation Inducing Ligand, or APRIL: TNF-family ligand implicated in regulation of B cell-mediated immune responses Soluble factor that functions via binding to TACI and BCMA receptors Critically drives IgA class switching through TACI Survival of IgA-producing plasma cells through BCMA Binding site BION-1301 APRIL Blocking APRIL is a distinct approach to reduce circulating levels of IgA, Gd-IgA, anti-Gd-IgA autoantibodies and immune complex formation Patients with IgAN have significantly higher levels of APRIL than healthy controls Higher APRIL levels in IgAN patients correlate with poor prognosis: Gd-IgA1, proteinuria and eGFR When APRIL is added to lymphocytes from IgAN patients, Gd-IgA1 significantly A polymorphism in the APRIL gene confers IgAN susceptibility Dulos J, et al. Blood 2016 128:2112;

APRIL Pathway Agents Uniquely Positioned in 4 Hit Hypothesis on the Pathogenesis of IgA Nephropathy BION-1301 should impact all four categories of IgAN pathogenesis, and in the future could potentially be used in combination with drugs that have different MOAs α-APRIL α-APRIL α-APRIL α-APRIL α-Complement indirect indirect Indirect/Non-Specific Competitors Blood pressure meds Immunosuppression (steroids) Adapted from: Suzuki, Clinical and Experimental Nephrology (2018), Barratt et al. Pediatr Nephrol (2018), Penfold et al. International Journal of Nephrology and Renovascular Disease 2018 modified.

BION-1301: First-in-Class Fully Blocking Anti-APRIL Antibody First-in-class monoclonal antibody blocking APRIL binding to both of its natural ligands, the BCMA and TACI receptors No toxicology findings observed in 1-month toxicology study Well-tolerated in clinical studies (NCT03340883, NCT03945318) Well-tolerated up to 2700 mg IV in a Phase 1 safety and PK/PD study of late stage multiple myeloma patients Animal studies demonstrate that treatment with BION-1301 reduces production of IgA, IgG and IgM

Human IgA+ Plasma Cells Express High TACI and BCMA Levels and Depend on APRIL for Survival and IgA production In vitro-generated IgA+ plasma cells selectively express high BCMA and TACI In vitro-generated IgA+ plasma cells require APRIL to survive and produce IgA Anti-APRIL (.01A) inhibits plasma cell survival and IgA production TACI BCMA BAFF-R Representative donor (N=6) Isotype control IgA+ Plasma cells IgG+ Plasma cells Viability plasma cells IgA production

Preclinical Data in Mouse Models Provide Compelling Rationale for BION-1301 in IgA Nephropathy Increased IgA production seen in hAPRIL Tg mice The BION-1301 parental antibody hAPRIL.01A inhibits IgA production in hAPRIL Tg mice Treatment with anti-APRIL mAb 4540 suppresses proteinuria in IgAN ddY mice* hAPRIL Transgenic Mouse Model ddY IgAN Mouse Model *Myette JR et al. A Proliferation Inducing Ligand (APRIL) targeted antibody is a safe and effective treatment of murine IgA nephropathy. Kidney International (2019) 96, 104–116

Preclinical Data from Tox Studies in Non-Human Primates (NHPs) Provide Compelling Rationale for BION-1301 in IgA Nephropathy BION-1301 Reduces Serum IgA, IgG and IgM levels in NHPs BION-1301 Dose Response in NHPs Dulos J, et al. ASH 2017 poster presentation: abstract 1827.

Phase 1 Study of BION-1301 in Healthy Volunteers & IgA Nephropathy Patients Ongoing Up to 5 Dose Cohorts Part 1 in Healthy Volunteers: Double-blind, Placebo-controlled, Single Ascending Dose 3 Dose Cohorts Part 2 in Healthy Volunteers: Double-blind, Placebo-controlled, Multiple Ascending Dose Part 3 in IgAN Patients: Open Label, Multiple Dose 1 Dose Cohort Primary Objectives: Assess safety profile in healthy volunteers (HVs) & IgAN patients PK/PD relationship in HVs & IgAN patients Establish proof-of-mechanism: Reduction in IgA in HVs and/or reduction in Gd-IgA in IgAN patients

FDA Co-Authored Article Supporting Reduction in Proteinuria as a Surrogate Endpoint for eGFR for Accelerated Approval Proteinuria As of January 2019, FDA officially agrees to proteinuria as surrogate endpoint for eGFR in IgA Nephropathy Expect other global health authorities to follow suit General consensus among KOLs (several involved in Ph3 discussions with FDA) that a 30% reduction in proteinuria is necessary for approval Estimated Glomerular Filtration Rate (eGFR) Ongoing discussion between industry and FDA on exactly how to measure a reduced rate of eGFR as endpoint Expecting publication to address this in early 2020

Several Ongoing Toxicology Studies to Support Long-Term Subcutaneous Dosing 1-Month intravenous (IV) toxicology study completed prior to IND filing No tox findings noted 1-Month subcutaneous (SC) local tolerability toxicology study completed Will support initiation of Phase 1 IV/SC bridging study in healthy volunteers 3-Month IV toxicology study completed Will support extending treatment of IgAN patients to 3 months in ongoing Phase 1 study 6-Month IV toxicology study To support Phase 2 study start

Business Overview

Aduro Strategic Collaborations and Partnerships ADU-S100 and other STING agonists cGAS-STING pathway inhibitor program Anti-CD27 agonist Oncology Autoimmune & Inflammatory Oncology $700M upfront & potential milestones $50M equity Co-development & co-commercialization $12M upfront $620M potential development and commercial milestones per product Research funding $447M potential milestones Global license Aduro leads US sales Profit/expense share US, major EU and Japan Royalties ROW Lilly responsible for global commercialization Single to low-double digit royalties Option to co-fund clinical development in exchange for increased royalties Mid single-digit to low teens royalties

Strong Financial Position and Broad Intellectual Property Portfolio Q3 2019 Financials Cash, cash equivalents and marketable securities $235.4M R&D expenses $15.5M G&A expenses $8.7M Shares outstanding as of September 30, 2019 80.5M Extensive Patent Portfolio Global Rights (includes in-licensed patents) >150 issued composition and methods patents >250 pending applications Nominal Expiration STING: 2025-39 APRIL: 2030-36 Corporate restructuring in Q1 2020 is expected to reduce operating expenses & extend cash runway

Potential Upcoming Catalysts Indication Catalyst H1 2020 H2 2020 ADU-S100 (MIW815) Intratumoral STING Agonist + spartalizumab (α-PD-1) Advanced/metastatic solid tumors or lymphomas Report complete Ph 1 dose escalation & enrichment results + pembrolizumab (α-PD-1) 1st-line SCCHN Report interim study results ADU-S100 (MIW815) Intravesical Relapsed/refractory NMIBC Initiate Ph 1b dose escalation study BION-1301 Anti-APRIL Antibody IgA nephropathy Report Ph 1 data in healthy volunteers Report Ph 1 data in IgA nephropathy patients Expected Timing SCCHN = squamous cell carcinoma of the head and neck NMIBC = non-muscle invasive bladder cancer

